Reagent components
Solution A: Calcein-AM 2mM 50uL in DMSO; ·Solution B: PI (1.5mM) 150uL in water
Product Description
Calcein AM /PI Double Staining Kit is utilized for simultaneous fluorescence staining of viable and dead cells. This kit contains Calcein-AM and Propidium Iodide (PI) solutions, which stains viable and dead cells, respectively (Fig. 1). Calcein-AM, an acetoxymethyl ester of calcein, is highly lipophilic and cell membrane permeable. Though Calcein-AM itself is not a fluorescent molecule, the calcein generated from Calcein-AM by esterase in a viable cell emits a strong green fluorescence (excitation at 490 nm, emission at 515 nm). Therefore, Calcein-AM only stains viable cells. On the other hand, PI, a nuclei staining dye, cannot pass through a viable cell membrane. It reaches the nucleus by passing through disordered areas of dead cell membrane, and intercalates with the DNA double helix of the cell to emit red fluorescence (excitation: 535 nm, emmision: 617 nm). Since both calcein and PI-DNA can be excited with 490 nm, simultaneous monitoring of viable and dead cells is possible with a fluorescence microscope. With 545 nm excitation, only dead cells can be observed (Fig. 1). Since optimal staining conditions differ from cell line to cell line, we recommend that a suitable concentration of PI and Calcein- AM be individually determined. Please note that PI is suspected to be highly carcinogenic; careful handling is required.
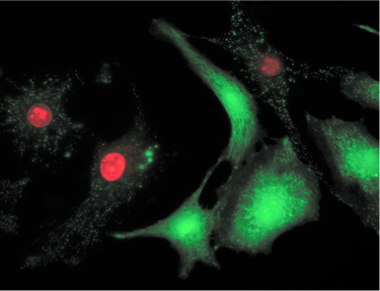
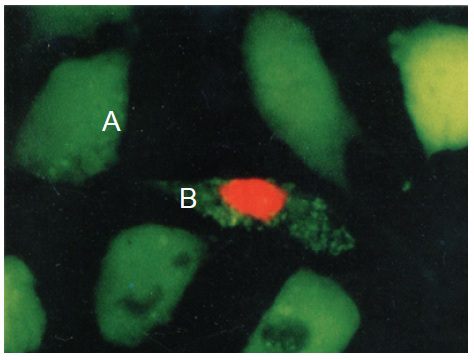
Fig. 1 Cell staining with Double Staining Hela cell, incubated with assay solution for 15 min. A) viable cell; B) dead cell
Required Equipment and Materials
Microscope with 490 nm excitation filter and 530 nm emission filter, CO2 incubator, 10 μl and 200 μl adjustable pipettes, PBS
Solution A (Calcein-AM);Solution B (PI) Storage Condition: -20oC ;Shipping Condition: blue ice
Application: Assay Procedure
1) Add 5 μl Solution A and 15 μl Solution B to 5 ml PBS to prepare assay solution.*
2) Wash the cell with PBS several times to remove residual esterase activity.
3) Add 100 μl of assay solution to 200uL 105 ~106 CELLS solution and incubate the mixture at 37 oC for 15 min.
4) Detect fluorescence using a fluorescence mircoscope with 490 nm excitation for simultaneous monitoring of viable and dead cells. With 545 nm excitation, only dead cells can be observed.
* The concentration of each reagent should be optimized. The following steps may be necessary to determine the suitable concentration of each reagent:
1.Prepare dead cells by 10 min incubation in 0.1% saponin or 0.1-0.5% digitonin or by 30 min incubation in 70% ethanol.
2.Stain dead cells with 0.1-10 μM PI solution to find a PI concentration that stains the nucleus only, not the cytosol.
3.Stain dead cells with 0.1-10 μM Calcein-AM solution to find a Calcein-AM concentration that does not stain the cytosol. Then stain viable cells with that Calcein-AM solution to check whether the viable cell can be stained.
Reference
1.E. S. Kaneshiro, et al., J. Microbiol. Methods, 17, 1 (1993);
2.L. S. De Clerck, et al., J. Immunol. Methods, 172, 115 (1994);
3.N. G. Papadopoulus, et al., J. Immunol. Methods, 177, 101 (1994);
4.M. Adler, et al., Neurotoxicology, 20, 571 (1999);
5.P. G. Bush, et al., Osteoarthritis Cartilage, 13, 54 (2005).